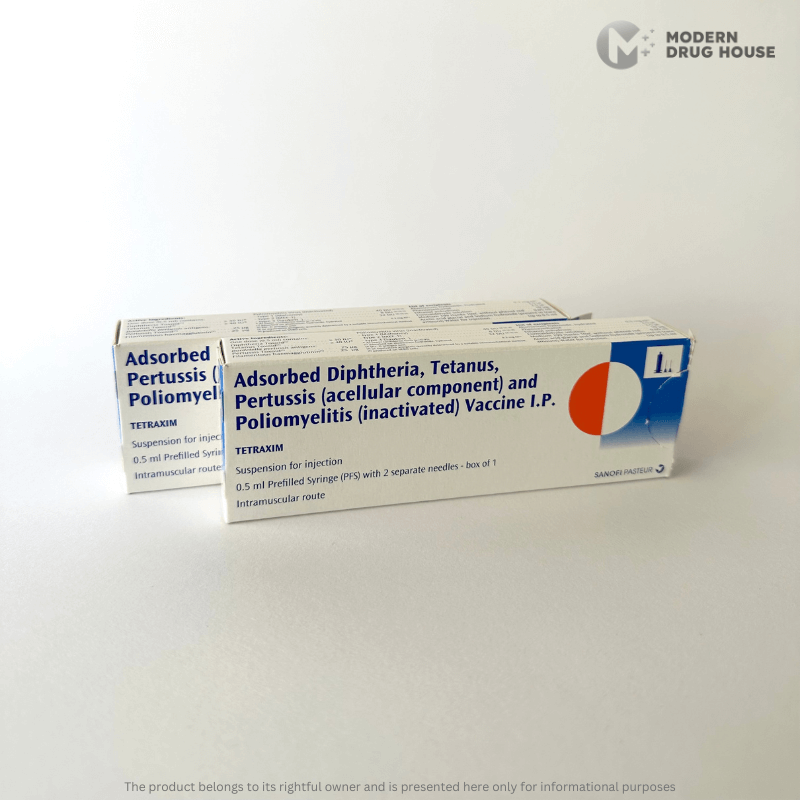
Tetraxim 0.5 ml
Adsorbed Diphtheria, tetanus, pertussis (acellular component) and Inactivated Poliomyelitis Vaccine I.P.
Composition
One dose (0.5 mL) contains: Diphtheria toxoid (1) (≥ 30 IU), Tetanus toxoid (1) (≥ 40 IU*),Bordetella pertussis antigens: Pertussis toxoid (1) (25 micrograms), Filamentous haemagglutinin(1)(25 micrograms). Poliomyelitis virus (inactivated)- (type 1 (Mahoney strain) is 40 DU(2) (3) (4)
, type 2 (MEF-1 strain) is 8 DU (2) (3) (4), type
3 (Saukett strain) is 32 DU (2) (3) (4)).
(1) adsorbed on aluminium hydroxide, hydrated 0.3 mg Al3+
(2) DU: D antigen unit.
(3) or equivalent antigenic quantity determined by a suitable immunochemical method.
(4) produced on VERO cells.
* As lower confidence limit (p=0.95)
TETRAXIM may contain traces of glutaraldehyde, neomycin, streptomycin and polymyxin B.
Manufactured / Marketed by
Sanofi Pasteur
Storage
Store at 2°C to + 8°C | Do not freeze.