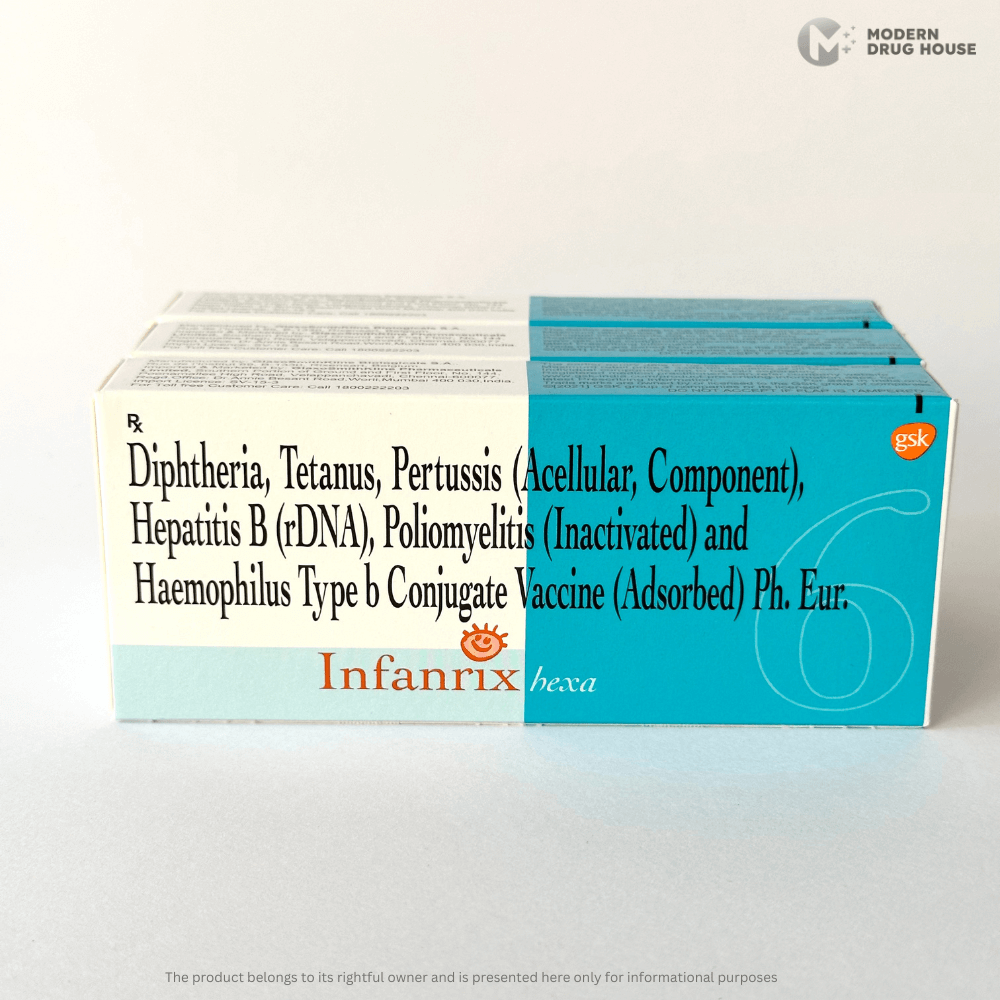
Infanrix Hexa 0.5 ml
Diphtheria, Tetanus, Pertussis (Acellular, Componentl) Hepatitis B (rDNA), Poliomyelitis (Inactivated) and Haemophilus Type b Conjugate Vaccine (adsorbed) Ph. Eur.
Composition
0.5 ml dose contains ≥30 IU diphtheria toxoid, ≥40 IU tetanus toxoid, 25 µg pertussis toxoid, 25 µg filamentous haemagglutinin,
8 µg pertactin, 10 µg recombinant hepatitis B surface antigen ( HBsAg ), 40 D-antigen units inactivated poliovirus type 1 (Mahoney), 8 D-antigen units inactivated poliovirus type 2 (MEF-1). 40 D-antigen units inactivated poliovirus type 1 (Mahoney) 8 D-antigen units inactivated poliovirus type 2 (MEF-1) 32 D-antigen units inactivated poliovirus type 3 (Saukett) etc.
Manufactured / Marketed by
GlaxoSmithKline Biologicals S.A. / GlaxoSmithKline Pharmaceuticals Ltd.
Storage
Store at + 2°C to + 8°C | Do not freeze. Protect from light.